no of electrons in f orbital|Atomic Orbitals : Pilipinas The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals.
Download and install BlueStacks App Player Windows PC 4.240.30.1002. Run Android apps on your PC
PH0 · The periodic table, electron shells, and orbitals
PH1 · Shells, subshells, and orbitals (video)
PH2 · How many electrons can an orbital of type f hold?
PH3 · How many electrons can an f orbital have?
PH4 · For s, p, d, and f orbitals, how many electrons can each hold?
PH5 · Electronic Orbitals
PH6 · Electron Orbitals & Orbital Shapes
PH7 · Atomic orbital
PH8 · Atomic Orbitals
PH9 · 2.2: Atomic Orbitals and Quantum Numbers
GentlyPerv's Porn Videos And Images, Gifs, Leaks ., Welcome in my exhibitionist life. Follow me in my pervy adventures, live my trips around the world with my cock always out!!!
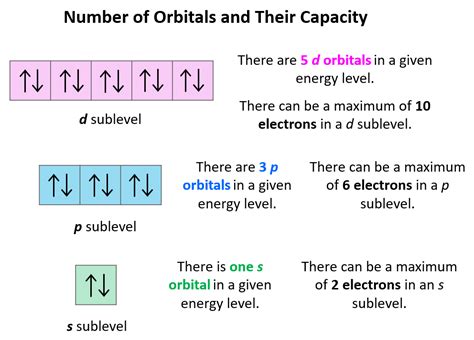
no of electrons in f orbital*******This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons.
Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired .As shown by the graphs, electrons of the s orbital are found closer to the nucleus .
How many electrons can an orbital of type f hold? A. 6. B. 10. C. 2. D. 14. E. 1. Since there can be [-ℓ, ℓ] orientations and since the orbital type f has ℓ = 3, we should have 7 .Atomic Orbitals The m l degeneracy is the number of orbitals within an l subshell, and so is 2l + 1 (there is one s orbital, three p orbitals, five d orbitals, seven f orbitals, and so forth). The .
The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals.The Pauli exclusion principle states that no two electrons in an atom can have the same values of all four quantum numbers. If there are two electrons in an orbital with given values for three quantum numbers, (n, .
The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum: s: 1 orbital, 2 .In completely occupied atomic orbitals, i.e. the atomic orbitals containing two electrons, each of the electrons has an equal and opposite spin when compared to the other. .Each orbital can hold two electrons. They are also known as atomic orbitals. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. We . The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and .The following equation summarizes these relationships and is based on the hydrogen atom: ΔE = Efinal −Einitial = −2.18 ×10−18 ( 1 n2f − 1 n2i) J Δ E = E final − E initial = − 2.18 × 10 − 18 ( 1 n f 2 − 1 n i 2) J. The values nf and ni are the final and initial energy states of the electron. The principal quantum number is . Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The number "1" represents the fact that the orbital is in the energy level closest to the nucleus. The letter "s" indicates the shape of the orbital: s orbitals are spherically symmetric around the nucleus— they look like hollow balls made of chunky . The number of orbitals in s, p, d, f are \[1,{{ }}3,{{ }}5,{{ }}7\] which are the first positive odd integers on the number line. Now, we multiply each integer by \[2\] to get the number of electrons. In this way, s subshell has two electrons, p subshell has six electrons, d subshell has ten electrons and f subshell has fourteen electrons in total.
s-orbitals can hold 2 electrons, the p-orbitals can hold 6 electrons. Thus, the second shell can have 8 electrons. The n=3 (third) shell has: The 3s orbital; The 3p orbitals; The 3d orbitals; s-orbitals can hold 2 electrons, p-orbitals can hold 6, and d-orbitals can hold 10, for a total of 18 electrons. Therefore, the formula $2n^2$ holds!Orbitals can be thought of as containers for electrons, and have different shapes. Orbitals are divided into s, p, d, and f orbitals according to their characteristic shape. Any one orbital can contain no more than two electrons.
no of electrons in f orbitalTo draw the orbital diagram, begin with the following observations: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals. According to Hund’s rule, all orbitals will be singly occupied before any is doubly occupied .Figure 9.6.9 9.6. 9: Orbital filling diagrams for hydrogen, helium, and lithium. According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium.Solution. Verified by Toppr. For f subshell azimuthal quantum number ( l = 3 ). Therefore permissible value of magnetic quantum number (m) are +3,+2,+1,0,−1,−2,−3. This f subshells possess seven orientations in space i.e. f subshells contain seven orbitals. Therefore maximum number of electrons in 4f orbital is 14. Was this answer helpful?
Q. Calculate the Z eff on the electron present in 5 d − orbital of G d (Atomic number = 64). Q. Maximum number number of electron that may be present on a 4 f − orbital is: Q. Number of lone pair of electrons present on the central atom of N O − 2 ion is?The four chemically important types of atomic orbital correspond to values of l = 0, 1, 2, and 3. Orbitals with l = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 and l = 0 contain one or more nodes.
Figure 9.6.9 9.6. 9: Orbital filling diagrams for hydrogen, helium, and lithium. According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium.The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons. As stated, the . 1 Answer. The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons. 2 electrons ---> see below: The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons.What Are F Block Elements? Elements whose f orbital gets filled up by electrons are called f block elements. These elements have electrons (1 to 14) in the f orbital, (0 to 1) in the d orbital of the penultimate energy level and in the outermost orbital. There are two series in the f block corresponding to the filling up of 4f and 5f orbitals .

The Azimuthal quantum number for the valence electrons of Ga^+ ion is ... (Atomic number of Ga = 31) asked Aug 3, 2021 in Chemistry by Kanishk01 ( 44.9k points)no of electrons in f orbital Atomic Orbitals When fluorine atoms are excited, then fluorine atoms absorb energy. As a result, an electron in the 2p y orbital jumps to the 3s orbital. Therefore, the electron configuration of fluorine(F*) in an excited state will be 1s 2 2s 2 2p x 2 2p y 1 2p z 1 3s 1. Fluoride ion(F –) electron configuration
The question specifically ask that no.of electron an orbital of f subshell can hold.. As we know that f subshell contain 7 orbital and each orbital can hold maximum 2 electons so correct answer would be 2..we can simply understand this by taking the real life example imagine that there is house named f which consists of 7 rooms so similarly in this case .
Local SEO strategies are needed to draw new customers to your website. It's not about attracting the most customers, but attracting the right ones that are close to you and can convert from a visitor to a buyer. LOCALSEO.PH. We put you on the Map! Get found and connect to customers in your community, We believe the push for LOCAL SEO to be a .
no of electrons in f orbital|Atomic Orbitals